Lupine Publishers | Journal of Cardiology & Clinical Research
Abstract
Introduction and Objective: Recent research has determined
that the “cardiac suction” phase occurs between systole and
diastole. The aim of this work was to analyze the suction capacity of
the left ventricle after excluding the right ventricle through an
atriopulmonary bridge.
Methods: An atriopulmonary bridge was performed on six dogs,
followed by right coronary artery occlusion to generate right
ventricular dysfunction. Cardiac output (CO), cardiac index (CI),
systolic index (SI), left ventricular stroke work index (LVSWI),
right ventricular stroke work index (RVSWI), systemic vascular
resistance (SVR) and pulmonary vascular resistance (PVR) were
evaluated using a Swan Ganz catheter. Recordings were acquired at
baseline before any procedure, 60 minutes after right coronary
occlusion with the atriopulmonary bridge connection closed and 60
minutes after opening the atriopulmonary connection. At the
end of the experiment, coronary angiography and histological examination
were performed to verify the right coronary occlusion.
Results: Sixty minutes of coronary occlusion produced decreased CO (3.43 to 2.25 l/min), CI (5.22 to 3.39 l/m2), SI (41.5 to 20.8
ml/beat/m2), LVSWI (53.3 to 14.4 g × m/beat/m2), and RVSWI (1.61 to -1.97 g × m/beat/m2). When the atriopulmonary bridge was
opened, CO increased to 3.39 l/min (p< 0.05), CI to 4.95 l/m2 (p< 0.05), SI to 45.1 ml/beat/m< sup>2 (p< 0.05), LVSWI to 40.8 g × m/beat/
m2 (p< 0.05) and RVSWI to 1.57 g × m/beat/m2 (p< 0.05), and right atrial pressure decreased from 10.6 to 3 mmHg (p< 0.05) and
PVR from 109 to 48.9 dyn/s/cm-5 (ns).
Conclusion: As demonstrated by means of an atriopulmonary bridge, right ventricular dysfunction experimentally induced by
ischemia is compensated by a left ventricular suction mechanism, restoring normal circulatory parameters.
Keywords: Ventricular suction; Atriopulmonary Bridge; Fontan; right ventricular exclusion; isovolumic diastolic phase.
Abbreviations: CI: Cardiac index; SI: Systolic index; LVSWI: Left ventricular stroke work index; RVSWI: Right ventricular stroke
work index; SVR: Systemic vascular resistance; PVR: Pulmonary vascular resistance
Recent studies have demonstrated an active suction phase
between systole and diastole [1-4], with muscle contraction, energy
expenditure and decreased intraventricular pressure. This active
suction generates a gradient between peripheral and ventricular
pressures, allowing blood flow into the heart [5-10]. Circulation
is a dynamic process in which the heart, the vascular system and
resistances modify their properties adapting according to the
circulatory needs. From the point of view of blood oxygenation
(though not histologically), we consider the systemic venous
system, the right ventricle and the pulmonary artery as belonging
to the venous system, while the pulmonary veins, the left ventricle
and the arteries make up the arterial system. The systemic and
pulmonary capillaries connect both systems, assuming that the
filling pressures are similar in both sides (venous and arterial).
Central venous pressure and pulmonary capillary pressure are
comparable, the latter being the load the venous system offers to the left ventricle. The small difference between peripheral
and heart pressure needs a suction energy in the left ventricle to
generate intraventricular depression.
Although both ventricles manage similar volumes, their
pressures are different. The left ventricular suction energy explains
right ventricular filling. In the present study, we experimentally
performed an atrioventricular bridge and generated right
ventricular dysfunction, leaving the left ventricle as the only
contractile component. In these conditions of right ventricular
dysfunction, we analyzed whether the atriopulmonary bridge
connection improved the hemodynamic conditions based on the
left ventricular suction mechanism.
The present study was conducted at Universidad de Avellaneda
in Buenos Aires (Argentina), after approval by the institutional
Ethics Committee. Six adult mongrel dogs slated for euthanasia, with
mean weight of 17 kg and 0.72 m2 (range 0.56-0.96) body surface
area, were used in the study. The experiments were performed
under “Arrive” regulations and according to the United Kingdom
1996 Act on scientific procedures on animals, the EU Directive
2010/63/EU for experimental animals and the “National Institutes
of Health” guide for the care and use of laboratory animals (NIH
Publication No. 8023, updated in 1978). The animals were sedated
with ketamine (10mg/kg) and femoral artery and vein cannulation
was performed for fluid infusion and arterial and central venous
pressure monitoring. Ventilation was controlled with intermittent
positive pressure using a Taka-Vent 550 respirator with 100%
oxygen, airway flow of 9 l/min and positive pressure of 11 cm H2O.
Anesthesia was maintained with 0.5-2% enflurane and fentanyl (5
mg/kg).
Surgical Protocol
Heparin (1 mg/kg) was administered after median sternotomy
and pericardiectomy. The atriopulmonary bridge connection was
performed between the right atrial appendage and the pulmonary
artery (through partial clamping) with an 8 mm diameter woven
conduit. A Swan-Ganz catheter was inserted into the pulmonary
artery, connected to a transducer and two recording channels, and
a second catheter was placed in the right atrium. Cardiac output
was assessed using the thermodilution technique.
Variables Recorded: Right atrial pressure, pulmonary artery
pressure, pulmonary capillary pressure, mean arterial pressure,
heart rate and cardiac output were recorded.
Calculations and formulas:
Cardiac index (CI): cardiac output/body surface area=l/min/m2
Systolic index (SI): CI/heart rate=ml/beat/m2
Left ventricular stroke work index (LVSWI): (mean arterial
pressure-pulmonary capillary pressure) × SI × 0.0136=g × m/
beat/m2
Right ventricular stroke work index (RVSWI): (pulmonary
arterial pressure-right atrial pressure) × SI × 0.0136=g × m/
beat/m2
Systemic vascular resistance (SVR): (pulmonary arterial
pressure-right atrial pressure)/cardiac output × 80=dyn/s/
cm-5
Pulmonary vascular resistance (PVR): (pulmonary arterial
pressure- pulmonary capillary pressure/cardiac output ×
80=dyn/s/cm-5
Measurements were taken at baseline before any procedure.
Following right coronary occlusion by ligation at its origin, volume
overload with saline was achieved to a threefold increase of right
atrial pressure. After 60-minute ischemia, the atriopulmonary
bridge was opened and kept patent during another 60 minutes.
Hematocrit, pH, pO2 and pCO2 were controlled at all times. The
experiment was concluded after 120 minute-ischemia and the
hearts were explanted. A coronary angiography was performed
to verify the right coronary artery occlusion, and tissue samples
were taken and fixed in 10% formalin for histological analysis. The
right ventricle was sectioned, taking samples at different heights of
the wall and additional samples near the left apex. Samples were
processed in an autothecnicon tissue processor and stained with
hematoxylin-eosin, periodic-acid Schiff (PAS) for glycogen and
basic fuchsin dye for necrosis-ischemia.
Statistical Analysis
Analysis of variance for randomized block design, Tukey test for
multiple comparisons and Friedman’s test for non-parametric and
post-hoc comparisons were used to analyze the data. Differences
with p<0.05 were considered as significant.
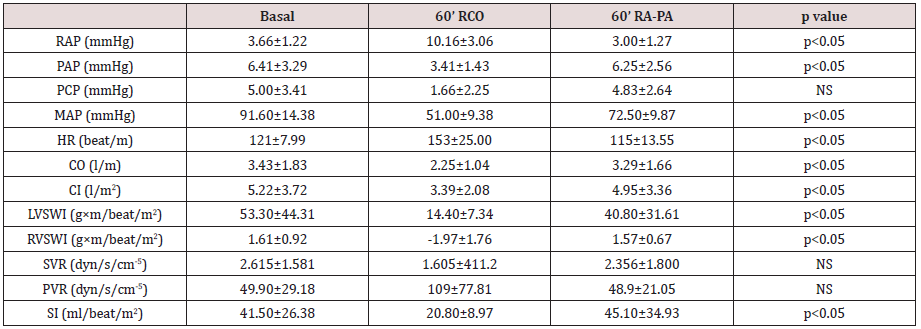
Table 1 shows hemodynamic values at baseline, at 60
minutes of right coronary artery occlusion and at 60 minutes
after atriopulmonary bridge connection. Both the right and
left ventricles showed no changes in myofibrillar architecture,
nucleus or transverse striation with hematoxylin-eosin staining.
The PAS technique evidenced marked glycogen reduction in right
ventricular sections, expressed as complete lack of magentacolored
granules, almost throughout the entire wall thickness.
Only a thin band of subepicardial parallel fibers and other isolated
fibers in the papillary muscles presented scarce glycogen granules.
Conversely, the left ventricle showed visible glycogen content. The
almost massive loss of intracellular glycogen demonstrated by the
PAS technique is due to the early change (first 30 minutes) observed
in experimental animals with coronary occlusion.
As confirmed by different studies, acute occlusion of the right
coronary artery produces right ventricular dysfunction [11]. Table
1 reflects the decrease of RVSWI (1.61 to -1.97 g × m/beat/m2) and of cardiac output (3.43 to 2.25 l/min) following right coronary
artery occlusion. The right ventricle expanded, increasing its atrial
pressure and decreasing pulmonary capillary pressure. At autopsy,
right ventricular dilation is frequent after its acute infarction.
Moreover, PVR increased from 49.9 to 109 dyn/s/cm-5 and the SI
decreased from 41.5 to 20.8 ml/beat/m2. The choice of 60 minutes
between coronary occlusion and atriopulmonary bridge opening
was not arbitrary. Previous works have established that a 40-minute
period of regional ischemia produces irreversible ventricular injury
[11]. In our case, histological analysis confirmed right ventricular
ischemia. It was seen that the injured right ventricle was not able to
behave as a simple conduit vessel, as volume overload worsened its
distention and impaired the SI (Table 1). All the animals evidenced
right ventricular damage, with contractile dysfunction and lack of
synchronization between both ventricles. Our model eliminated the
pericardial protective function, which in case of being intact would
have attenuated right ventricular dilation secondary to ischemia.
Table 1: Hemodynamic values.
RCO: right coronary occlusion; RA-PA: right atrium-pulmonary artery bridge; RAP: right atrial pressure; PAP: pulmonary arterial pressure; PCP: pulmonary capillary pressure; MAP: mean arterial pressure; HR: heart rate; CO: cardiac output; CI: cardiac index; LVSWI: left ventricular stroke work index; RVSWI: right ventricular stroke work index; SVR: systemic vascular resistance; PVR: pulmonary vascular resistance; SI: systolic index.

RCO: right coronary occlusion; RA-PA: right atrium-pulmonary artery bridge; RAP: right atrial pressure; PAP: pulmonary arterial pressure; PCP: pulmonary capillary pressure; MAP: mean arterial pressure; HR: heart rate; CO: cardiac output; CI: cardiac index; LVSWI: left ventricular stroke work index; RVSWI: right ventricular stroke work index; SVR: systemic vascular resistance; PVR: pulmonary vascular resistance; SI: systolic index.
In view of the expected post-ischemic right ventricular
dilation (worsening after increasing preload) we applied Fontan´s
principle: right atrial connection to the pulmonary artery, avoiding
the dysfunctional right ventricle [12]. We sought to increase left
ventricular preload and relieve right ventricular distention. Right
ventricular ischemia reduced cardiac output to 65% and LVSWI to
27% their baseline values. Opening of the atriopulmonary bridge
connection after 60-minute ischemia produced hemodynamic
recovery by unloading the right atrium into the pulmonary artery.
Cardiac output improved to 95% its baseline value, recovering to
3.29 l/min (p<0.05), SI to 45.1 ml/beat/m2 (p<0.05), LVSWI to
40.8 g × m/beat/m2 (p<0.05) and RVSWI to 1.57 g × m/beat/m2
(p<0.05). Also, right ventricular pressure decreased from 10.16 to
3 mmHg (p<0.05) and PVR from 109 to 48.9 dyn/s/cm-5 (ns). The
new scenario that emerges after opening the atriopulmonary bridge
connection by eliminating the passage through the dysfunctional
right ventricle, transfers the right circulatory mechanism exclusively
to the left ventricular suction capacity. Our experimental model
shows how the obstacle of a dysfunctional right ventricle can be
overcome bypassing this chamber with an atriopulmonary bridge,
with the sole prior condition of acceptable pulmonary resistances.
Thus, the left ventricular suction mechanism acts as a cardiac flow
engine.
The increase in SI was higher than expected, considering
that the pressure difference between the right atrium and the
pulmonary artery disappeared rapidly when the atriopulmonary
bridge connection was opened. It is possible that reduced right
preload conditions a slight improvement of the right ventricle
by not worsening the ischemic effects, thus contributing to the
increase in SI after a discrete functional recovery. This analysis is
concurrent with previous investigations [1-5] where the isovolumic
diastolic phase was studied as an active phenomenon generated by
a myocardial contraction that tends to expand the left ventricular
apex-base distance after the ejective phase, producing a suction
effect similar to that of a “plunger”. A drop in intraventricular
pressure is generated that, in turn, elicits ventricular suction. It is
an active process during the isovolumic phase, which is erroneously
considered as diastolic. When this pressure is sufficiently negative
(-10 mmHg) and the left ventricle is elongated and “uncoiled”, the
mitral valve opens with the ensuing rapid blood filling from the
atrium. This suction phase between systole and diastole of the
human cardiac cycle lasts between 100 and 120 ms with active
muscle contraction and a drop of intraventricular pressure below
zero, as shown by Tyberg [13] with balloon mitral valve occlusion
in the dog. Fontan procedures, with atriopulmonary bypass that
avoid the right ventricle, clinically show the efficiency of the left
ventricular suction mechanism. Its validity is confirmed even before
recent physio mechanical investigations. The same consideration
should be applied to left circulatory mechanics, where blood is
bypassed from the left ventricle into the aorta. It can be concluded
that suction due to the elastic recoil of the helical ventricular
structure is an active process. Myocardial contraction occurs during
the left ventricular isovolumic phase. In cases of ventricular dilation,
this suction mechanism (“plunger”) becomes more precarious. This
concept may help to establish a new evaluation of heart failure and
its clinical severity [14-16].
As demonstrated using an atriopulmonary bridge, right
ventricular dysfunction produced experimentally by ischemia is
compensated by a left ventricular suction mechanism, maintaining
normal circulatory parameters
Cardiac output (CO) is given by:

where E1: left ventricular energy; E2: right ventricular energy;
R1: systemic vascular resistance; and R2: pulmonary vascular
resistance.
E1/R1=E2/R2 shows the output energy and vascular
resistance ratio between both circulatory systems; then:
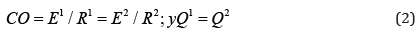
With Q1= left ventricular flow potential; Q2= = right ventricular
flow potential
Venous pressure is the same in both circulatory systems. If the
right ventricle is withdrawn from the circuit (E2=0), cardiac energy
after surgery results as:

For more Lupine Publishers Open Access Journals
Please visit our website
For more Journal of
Cardiology & Clinical Research Please Click Here:
To Know more Open
Access Publishers Click
on Lupine
Publisher
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
No comments:
Post a Comment