Lupine Publishers | Journal of Cardiology & Clinical Research
Introduction: A great number of the studies have shown that platelets play a role in closure of the PDA. However, studies that
reported that platelet parameters were not associated with PDA were also published. We also wanted to contribute to clarify the
relationship between PDA and platelet parameters.
Materials and Methods: Preterm infants that less than 34
gestational weeks were examined to echocardiography at the time
of detected clinical findings or within 24-72 h after admission to our
unit, routinely. The patients were divided into two groups
according to echocardiography findings randomly; hsPDA require ductal
closure treatment and non-hsPDA. The platelet count,
MPV, PDW, PCT and Platelet Mass Index values of both groups were
compared.
Results: There was no difference between the two groups in terms of MPV, Platelet count and Platelet mass index. However,
PDW and PCT were statistically significantly in the study group than the control group.
Discussion: As a result, according to our study, platelet count, MPV and platelet mass index cannot be used to predict either
hsPDA or treatment success, but a low PCT and high PDW can be used predict hsPDA but not treatment success.
Patent Ductus Arteriosus (PDA) can cause mortality and
morbidity such as respiratory distress syndrome (RDS), pulmonary
hemorrhage, bronchopulmonary dysplasia (BPD), intraventricular
hemorrhage (IVH), necrotizing enterocolitis (NEC), retinopathy
of prematurity (ROP) [1]. For this reason, early diagnosis and
treatment of PDA is the most important point. The main diagnostic
method of PDA is Doppler echocardiography [2]. However, there is
no clearly consensus on diagnosis of hemodynamically significant
patent ductus arteriosus (hsPDA). Therefore, new diagnostic
methods of PDA are needed. A great number of the studies have
shown that platelets play a role in closure of the PDA [3-6]. The
first of these studies, Echtler et al. studied the relationship between
ductal closure and platelet parameters in animals [3]. In the
same study, the ductus arteriosus did not close (thus, remained
permanently open) in animals in which platelet functions were
compromised. After this study, they studied on premature infants
about relationship between ductal closure and platelet parameters.
According to this study, a low platelet count and a low PDW were
risk factors for PDA. However, studies that reported that platelet
parameters were not associated with PDA were also published
[7-11]. We also wanted to contribute to clarify the relationship
between PDA and platelet parameters.
This observational, retrospective cohort study was conducted
between August 2017 and 2018. Preterm infants that less than 34
gestational weeks were examined to echocardiography at the time
of detected clinical findings or within 24-72 h after admission to
our unit, routinely [12]. The patients were divided into two groups
according to echocardiography findings randomly; hsPDA require
ductal closure treatment and non-hsPDA. hsPDA was defined when
at least one of the clinical findings associated with PDA was present:
a hyperdynamic precordium; a sustained murmur; tachycardia;
hypotension; oliguria; an increased pulse pressure; an increase in ventilation pressure and/or oxygen demand; and at least one
echocardiographic finding: ductal diameter ≥1.5mm, left atrium/
aortic root ratio ≥1.5, and/or diastolic flow failure in the abdominal
aorta or inverse flow. We applied intravenous or oral ibuprofen to
close the hsPDA. Intravenous or oral paracetamol was given in cases
who ibuprofen is unsuccessful or contraindicated. After treatment,
echocardiography was performed again, and the PDA was classified
as open or closed. We excluded those with conditions that might
cause inflammation or affect platelet count and/or function
(Antenatal steroid use, PPROM, early sepsis, chorioamnionitis,
congenital viral infections, preeclampsia), congenital heart disease,
pulmonary hypertension, perinatal asphyxia, congenital anomaly,
chromosomal anomaly, thrombocytopenia (<50.000/mm3), and lack
of data. Written informed consent was obtained from all parents.
All echocardiographic examinations were performed by Vivid S6
Echocardiography System fitted with a 10S transducer (General
Electric Healthcare, Milwaukee, WI, USA). Blood samples taken
from an umbilical venous catheter at between 48-72 hours and 7.
day, were collected in ethylenediaminetetraacetic acid-containing
tubes and blood counts performed using a Coulter Counter model
LH (Coulter Electronics, Hialeah, FL, USA). This yielded the platelet
count, MPV, PDW, PCT. The platelet mass index was obtained from
the platelet count (103/mm3) and the MPV (fL). We recorded
gestational age, birth weight, sex, mode of delivery, Apgar scores
(at 1 and 5 min) 48-72 h and 7. day platelet parameters, surfactant
requirement, ventilation history, IVH, NEC, ROP, BPD, duration of
hospitalization and any death.
Statistical analyses were performed using SPSS for Windows
ver. 22.0 (SPSS Inc., Chicago, Illinois). The paired samples t-test
and independent samples t-test were used to compare continuous
variables. Continuous variables are presented as means ± SDs, and
categorical variables are given as frequencies with percentages. A
p-value less than 0.05 was considered statistically significant.
258 newborns under 34 weeks were admitted to our
unit, of whom 121 were excluded. The study group consisted
of 72 premature infants with hsDPA who applied ductus
closure treatment and 65 premature infants without hs DPA or
spontaneously closed PDA consisted of the control group (Figure
1). The demographic characteristics of both groups are shown in
Table 1. The mean gestational age and the mean birth weight of the
study and control groups were, respectively, 31.4±3.8 vs. 32.3±4.5
weeks (p=0.12); 1441±347 vs. 1,539±286g (p=0.08). There was
no difference between two groups in perinatal parameters. The
platelet parameters of both groups are shown in Table 2. There
was no difference between the two groups in terms of MPV, Platelet
count and Platelet mass index. However, PDW and PCT were
statistically significantly in the study group than the control group.
Table 1: Comparison perinatal characteristics of the study and control groups.
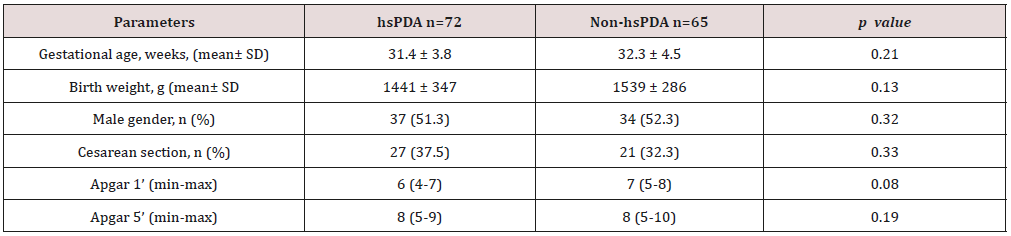
hsPDA: hemodynamically significant patent ductus arteriosus.
Table 2: Comparison of the platelet parameters of the study and control groups.
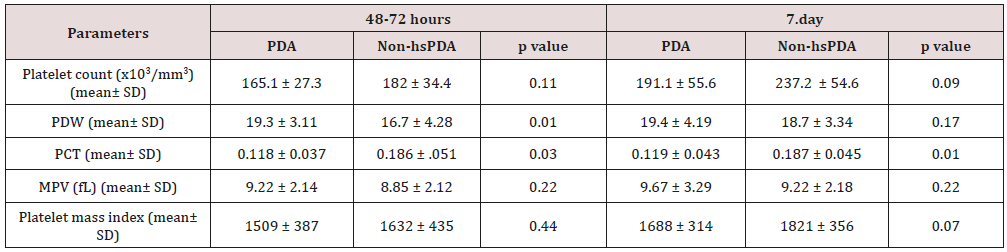
PDW: platelet distribution width; PCT: platocrit; MPV: mean platelet volume; Platelet mass index: the platelet count (103/mm3) X
MPV (fL); hsPDA: hemodynamically significant patent ductus arteriosus.
Figure 1: Flowchart of study.

PPROM: Preterm premature rupture of the membranes; hsPDA: hemodynamically significant patent ductus arteriosus.
Low oxygen pressure, elevated prostaglandin and nitric oxide
levels are the main factors affecting continuity of the ductus
arteriosus in the uterus. After birth, increased oxygen levels and
decreased prostaglandin levels enable functional closure of the DA
[13]. In addition to this mechanism, different mechanisms of closure
of the ductus began to be discussed. The discussion began when
Echtler et al. showed that platelets were attached to the lumen of the
closed ductus arteriosus and confirmed this experimental finding
via a retrospective study of preterm births [3]. After this animal
study, various hypotheses about the role played by platelets in duct
closure in newborns have been proposed. The most acceptable
hypothesis is an effect of platelets on DA contraction, which occurs
immediately after birth in term newborns, triggering hypoxia in the
vessel wall by decreasing the blood flow in the venous lumen and
vasa vasorum; in preterm newborns, the cells in the ductus wall are
fed by the ductal lumen because of the absence of a vasa vasorum.
As the ductus wall is thin, contraction is inadequate and endothelial
damage and platelet aggregation thus develop because of vesselwall
hypoxia. Based on this hypothesis, it was claimed that platelet
counts were important in terms of DA closure in preterm infants, as
they are in the pathophysiology of adult vascular diseases [14,15].
However, this hypothesis is not supported by the fact that platelet
transfusion does not reduce the incidence of PDA in preterm
newborns with immune thrombocytopenia and does not increase
the PDA frequency in term newborns with severe thrombocytopenia
secondary to Wiskott-Aldrich syndrome [16-20].
In Fujioka et al. [21-23]. the platelet count was not related to
PDA diagnosis or treatment success. On the other hand, Echtler
et al. [3,5,6] reported that a low platelet count increased the
hsPDA incidence [24-25]. In some works performed after these
contradictory studies, it was reported that large platelets create a
greater potential risk of prothrombotic reactions; large platelets are
more aggregated than small and normal platelets given the greater
number of receptors such as thromboxane A2-B2 and glycoproteins
IIb-IIIa on the surfaces of large platelets. It was suggested that the
increased metabolic and enzymatic activities of dysfunctional
thrombocytes, rather than the platelet count, were associated with
PDA [26-29]. We sought to identify parameters related to platelet
function associated with PDA. These remain controversial; all of
MPV, PDW, PCT, and platelet mass index have been associated with
cardiovascular diseases in adults [30-35]. In addition, in a limited
number of studies on neonates, the MPV and PDW were shown to
be associated with prematurity complications such as RDS and BPD
[36-37].
In our study, no difference was found between the platelet
counts of the hsPDA and control groups at 48-72 h and 7. day. In
addition, there was no difference between the platelet counts of
newborn who did and did not fail treatment. In conclusion, the
platelet count was not a predictor of hsPDA diagnosis or treatment
success. The results of our study contradict those of the two major
meta-analyses conducted by Simon et al. and Mitra et al. but
support the cohort study of Sallmon et al. [18-20]. PCT was lower
and PDW was higher in the study groups than control groups and the
difference between the two groups was statistically significant.
However, MPV and platelet mass index were similar in both groups.
Thus, we conclude that the PCT and PDW can be used to predict
hsPDA but not treatment success. Demirel and Dizdar et al. [4].
reported that the PDW was higher in preterm infants with hsPDA
than in control groups [38,39]. Bekmez et al [40]. reported that
a low PCT increased the hsPDA incidence Demir et al.[41]. found
a high MPV and a low platelet mass in the hsPDA group. We also
excluded patients who received ibuprofen as ductus closure therapy
because of potential effects on platelet count and functions. Infants
born to mothers with prior pre-eclampsia, which affects platelet
count and ductal flow because of the increased placental resistance,
were also excluded [42-44]. We also excluded infants with perinatal
asphyxia associated with an increased PDA, thrombocytopenia, and
platelet dysfunction [45-47]. Newborns whose mothers had earlier
received steroids were excluded because of possible effects on the
platelet count. We thus excluded all pathologies that may affect
platelet count and function and induce inflammation. There were
some limitations of our study. The first limitation of our study is
that it was retrospective in nature. The second limitation is modest
sample size. As a result, according to our study, platelet count, MPV
and platelet mass index cannot be used to predict either hsPDA or
treatment success, but a low PCT and high PDW can be used predict
hsPDA but not treatment success.
For more Lupine Publishers Social Bookmarking Click on Below link
https://issuu.com/lupinepublishers-oaj/docs/lupine_publishers_social_bookmarking_sites.docx