Lupine Publishers | Journal of Cardiology & Clinical Research
Left ventricular non-compaction (LVNC) is a myocardial disorder,
classically defined as a double-layered myocardium, consisting
of a thick, spongy/hypertrabeculated, non-compacted endocardial segment
and a thin, compacted, epicardial portion. The
American Heart Association (AHA) classifies LVNC as a distinct primary
genetic cardiomyopathy, while the European Association
of Cardiology (ESC) as an unclassified cardiomyopathy. Despite the
magnitude of the entire literature yield on this topic, to date
the pathogenesis, prognosis, and treatment are still unclear. Prevalence
and mortality can range respectively from 0.05% to 0.26%
and 5% to 47%, but they are affected by the imaging criteria adopted for
the diagnosis. In fact, LVNC has been for years incidentally
discovered during autopsy of unexplained sudden cardiac deaths.
Conversely, with the advent of increasingly sophisticated
cardiac imaging techniques, the presence of hypertrabeculated myocardium
has become very common. Both echocardiographic
and magnetic resonance criteria have been proven to overestimate the
diagnosis, which shares a peculiar phenotype with other
pathologies. It is known that a hypertrabeculated left ventricle leads
to a symptomatic triad consisting of heart failure, arrhythmias
and thromboembolisms. Therefore, it is current opinion of the authors
that a “non-compaction cardiomyopathy” (NC-CMP) seems
to be the most comprehensive definition of a disease that, similarly to
the other cardiomyopathies, and regardless of its etiology,
beyond a peculiar phenotype shares a distinct symptomatology and
deserves to be listed as an entity between cardiomyopathies.
Keywords: Left Ventricle Non-Compaction; Non-Compacted Cardiomyopathy; Cardiomyopathies; Heart Failure; Sudden Cardiac
Death
Left ventricular non-compaction (LVNC) is a myocardial
disorder, classically defined as a double-layered myocardium,
consisting of a thick and spongy or hypertrabeculated endocardial
segment, defined as non-compacted, and a thin and compacted
portion, laying epicardially [1]. The above-mentioned hyper
trabeculations (HXTs) characteristically involve the left ventricle
(LV), especially the apex, the lateral, infero-lateral and inferior
wall [1,2], and less frequently the right ventricle [3]. Since its first
reports, LVNC has always been considered a controversial pathology
(Figures 1 & 2). In facts Grant and colleagues, for long accredited
as discoverers in 1926, presented a case of persistent sinusoids
instead of LVNC [1]. In addition, a lack of uniqueness among the
World Health Organization [4], the European Society of Cardiology
[5]. and the American Heart Association [6] for its classification,
and the presence of trabeculae as a terminal phenotype of LV
hemodynamic overload or other myocardial affections, has led
several authors to debate on the real existence of a true form of
uncomplicated primitive non-compacted cardiomyopathy [7,8].
Figure 1: Two different anatomo-pathological macroscopic sections of the left ventricle, presenting a non-compacted
myocardium. Modified from Lorca et al. Int J Cardiol 2016.
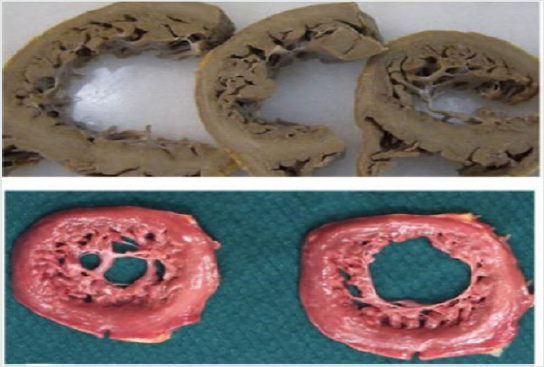
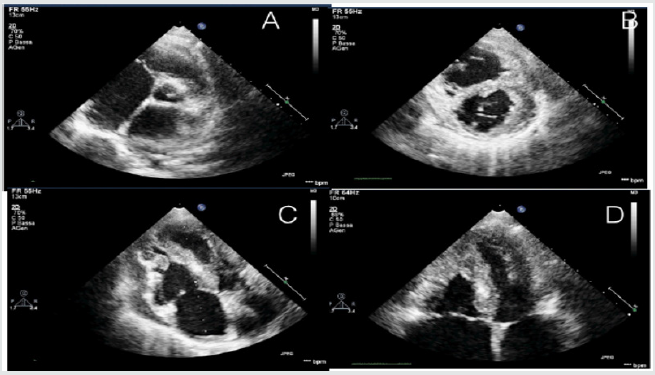
Figure 2: Echocardiographic features of a young adult of 16-years old, admitted in emergency room with acute signs and
symptoms of acute heart failure.
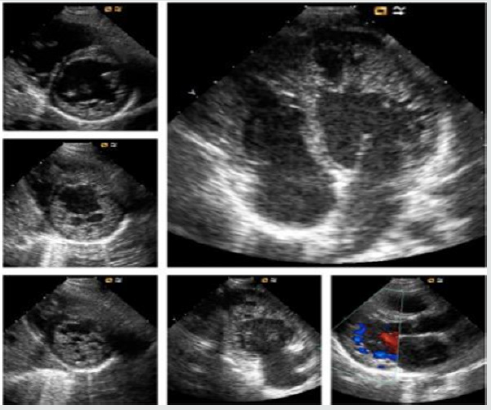
The primitive hypothesis concerning LVNC is that the presence
of HTXs is due to a failure in myocardial morphogenesis, in which the
immature, non-compacted fetal myocardium normally undergoes
a physiological compaction process during ontogenesis [1]. LVNC
may occur isolated, in familial forms or associated with several
congenital, genetic, neuromuscular and chromosomal conditions
[9]. Mutations in the sarcomere gene, particular in MYH7, are the
most common and non- sarcomere gene mutations (such as TAZ
and NOTCH1) have also reported [10-12]. Some LVNC individuals
have been detected by tracking asymptomatic relatives of affected
patients [9], and therefore, a close correlation between genotype
and phenotype has been recently underlined by 2 recent studies
using NEXT-generation sequencing [13,14]. All these pathogenic
variants were independent risk factors for cardiovascular events
[13]. In addition, mutations in hyperpolarization-activated cyclic
nucleotide channel 4 (HCN4) have also been reported in families
with sinus node dysfunction and LVNC [15,16].
Since only few studies focused on LVNC incidence, both
prevalence and mortality are challenging to assess [1]. Among
adults, it can be diagnosed in 0.05% - 0.26% of the cases, and
approximately 0.14% of pediatric patient, with an overall mortality
ranging from 5% to 47% in both populations [1]. Unfortunately, all
the reports are affected by the diagnostic criteria adopted, imaging
or autopsies, overestimating or underestimating the real prevalence
[1,17] .In addition, and similarly with others cardiomyopathies,
LVNC can be a subtle disease [17]; if not promptly diagnosed,
patients may be asymptomatic for a long time and the onset may
range from the early life to the adulthood [8].
The advent of increasingly sophisticated cardiac imaging
techniques set the spotlight on HXTs as a very common finding,
instead of the rare disease that was previously considered
[2,18,19]. Accordingly, Jenni, Chin and Stollberg defined different
echocardiographic criteria, while Petersen, Jacquier, Captur and
Stacey defined some cardiovascular magnetic resonance (CMR)
criteria. 1 Unfortunately, they both showed very poor specificity
[7,8], even if CMR overcame the ultrasound-related limits in
morphologic assessment, and demonstrated a thigh correlation
between late gadolinium enhancement myocardial fibrosis and
clinical severity of the disease [20]. Nevertheless, there still a lack
of an imaging-driven diagnostic gold standard [1,9].
Clinical findings are variable, including several grades of diastolic
and systolic dysfunction, heart failure (HF), thromboembolic
events, and malignant arrhythmias. 1 However, the most severe
outcome is the sudden cardiac death. Atrial fibrillation, right/left
bundle branch block, and repolarization abnormalities may be the
only electrocardiographic features present at the moment of the
diagnosis [2,8]. There is no specific therapy and LVNC management
depends on the clinical manifestations; anticoagulation is indicated
only if atrial fibrillation, heart failure, previous embolism, or intracardiac
thrombus formation are present [1,21].
LVNC has always been a controversial disease, with some
unresolved issues. First of all, there is a heterogeneous genetic
background and a wide spectrum of associated conditions that
may occur contextually with HTXs. Secondly, LVNC real prevalence
is still unknown. Certainly, the lack of any echocardiographic or
CMR diagnostic gold standard, with frequent overestimation/
underestimation, does not help to clarify all the uncertainties. In
addition, there are a wide variety of overlapping conditions that
may occur with secondary myocardial HXTs, for example, a dilated
cardiomyopathy or the end-stage hypertrophic cardiomyopathy
(Figure 3). On the other hand, it is common experience that not all
the above-mentioned cardiomyopathies and conditions can present
an end-stage non-compacted phenotype and generalizing this
aspect would be very simplistic. 8 In addition, Lorca and colleagues
recently reiterated that non-compacted forms of cardiomyopathy
do exist, especially during early stages of life, and they can be
demonstrated in some forms of unexplained sudden deaths. 17
Furthermore, a multicenter longitudinal prospective study [7],
despite its conclusions, and if carefully read between the lines,
suggests that a significant proportion of asymptomatic patients
meets all currently used imaging diagnostic criteria for LVNC.
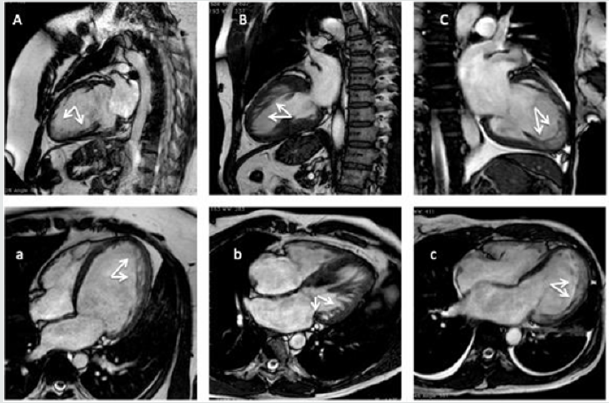
Figure 3: Cardiac magnetic resonance imaging (CMR) exams of patients matching the currently imaging criteria for CMR (A,
B, C, 2-chambers view; a, b, c, 4chambers view). (A, a) A 23-years-old male patients with a dilated cardiomyopathy. (B, b) a
38-years old woman with an end-stage hypertrophic cardiomyopathy. (C, c) a 45-years old woman admitted in emergency
room for acute heart failure, and history of silent cerebral infarcts.
However, they demonstrated that outcomes are increased by
symptoms and clinical conditions when associated with a noncompacted
phenotype. Indeed, a recently published multicenter
register provided an accurate estimation of genetic-phenotype
association, and clinical and events of LVNC patients, concluding
that the clinical course of symptomatic LVNC patient with a
genotype-phenotype matching can be severe [13,14]. Given all
these premises, it is reasonable considering the existence of a
primitive non-compacted disease, congenital, and a mild form,
with a late-onset, and/or acquired conditions. HF, thromboembolic
events, and malignant arrhythmias seem to constitute the clinical
triad for LVNC patients, and sudden cardiac death the most
severe outcome. There is a tight genotype-phenotype correlation,
with a wide spectrum of genes and mutation involved, as well
as hypertrophic cardiomyopathy, for example, and the current
imaging-derived diagnostic criteria probably need to be revised;
perhaps it should be worth to combine some imaging and clinical
criteria. At last, multicentric registries should be considered to
help the real prevalence assessment of non-compacted forms of
cardiomyopathies.