Lupine Publishers | Advancements in Cardiology Research & Reports
Abstract
Keywords: Heart Failure; Transplantation; Mechanical Circulatory Support
Twine and Twine or Lose the Plug- Dislodged Left Atrial Appendage Closure Device
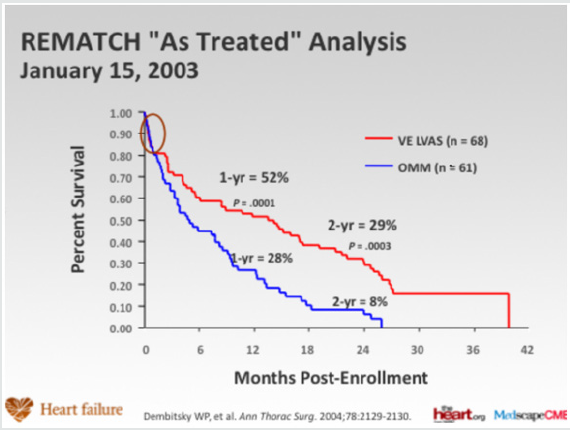
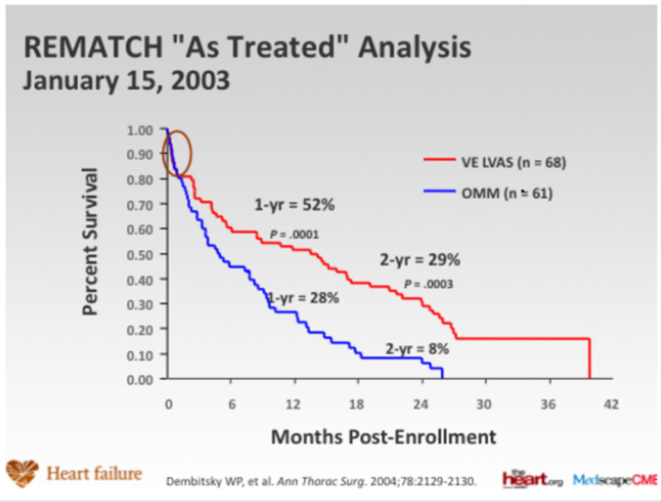
In the optimally medically treated control arm of the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial [1] which evaluated an externalized pulsation ventricular assist device, survival at one year was 28% and 6% at two years, underlying the poor prognosis of this clinical entity (Figure 1). Driving the need for mechanical circulatory support (MCS) is the relative paucity of donors and the unmet need for orthotopic heart transplantation in the general population. There has also been an increase in the number of patients who require mechanical circulatory support (MCS) as a bridge to transplantation [2]. This has been driven, particularly in the UK by limitation of the number of hearts for donation, and also to buy time on the transplant waiting list. This is due to an increase in the numbers of non-heart beating donors (DCDs), whereby retrieval takes place in a circulation arrested donor, and the increased survival of head injury patients and those with intracranial bleeds who are treated by a decompressive craniotomy, reducing the pool of donors who have raised intracranial pressure and who have coned, resulting in brain stem death. The net result is a retrieval rate for heart transplantation of around 19%. The risk of having preformed antibodies directed against the donor heart (sensitised patients) is increasingly likely and is particularly challenging as it may increase the risk of rejection and allograft vasculopathy. There has also been an increase in the number of patients requiring MCS as a bridge to transplantation [3]. This allows many severely ill adults and paediatric patients to survive until a suitable donor heart is available. Patients with MCS are at increased risk for rejection, infection, stroke, and bleeding. The need for transfusions also increases the risk of pre-sensitization [3-5]. Survival at 1 and 5 years is decreased in patients requiring MCS prior to transplantation, but still higher than 80% and 70%, respectively (ISHLT database) [2].
Figure 1: Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial
(Rose et al N Engl J Med 2001; 345:1435-1443).

Advances in Donor Allocation and Selection
Figure 2: Competing outcomes for continuous flow LVADS (82% survival at 1 year, intention to treat).
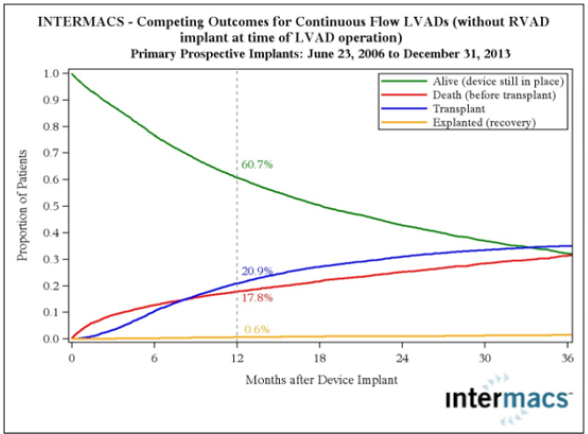
Transplantation eligibility is always considered with regard to
risk factors, especially, pulmonary hypertension (Figure 2). Right
heart catheterization must be performed in all potential candidates
for heart transplantation in order to quantify pulmonary vascular
resistance [7]. Right heart failure is a substantial cause of mortality.
Right ventricular failure is likely when post implant pulmonary
artery pressures exceed 50 mmHg. Patients with chronic heart
failure may develop pulmonary hypertension due to elevated left ventricular end diastolic pressure with elevated left atrial
and pulmonary venous pressures. This is a reactive form of
pulmonary hypertension and may fall when the cardiac output is
increased with inotropes or unloaded with nitrate infusions [7].
The transpulmonary gradient is calculated by subtracting the left
atrial filling pressure from the mean pulmonary artery pressure. A
fixed transpulmonary gradient in excess of 14 mmHg is associated
with greatly elevated risk, and thus this cut off is used in the UK [8].
In such patients a destination therapy strategy may be used with
continuous flow LVADS.
Mechanical Circulatory Assist Devices
Right ventricle failure is a leading cause of morbidity and death after LVAD implant (incidence of about 35%), and can be very difficult to predict [15,16]. Various means to assess right ventricle function both pre- and postoperatively have been assessed (10). Right ventricular failure risk scores have been created that stratify the risk of right ventricular failure (RVFRS) and death after LVAD implantation (Figure 3). One such RVFRS found independent predictors of right ventricular failure to include vasopressor requirement, aspartate aminotransferase >80 IU/L, bilirubin >2.0mg/dL and creatinine >2.3mg/dL [15]. Another study developed a score to predict RVAD need after LVAD placement, which included factors of cardiac index, right ventricular stroke work index, severe preoperative right ventricular dysfunction, creatinine, previous cardiac surgery and systolic blood pressure [16]. More recently the presence of severe TR and a tricuspid annulus of >43mm and right ventricular sphericity have been proposed as predictive of occult RV failure and need for biventricular support. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) registry, which follows all long-term MCS systems in the United States, has defined patient profiles that can help identify risks associated with the timing of implant [17]. In the future, the INTERMACS patient profile would be a useful tool to improve management and outcomes of patients who need VAD implant and unify criteria for future clinical trials and devices (Figure 4). As more LVAD patients are listed for heart transplant, a competition has occurred for organs between stable LVAD supported registrants and less stable registrants listed UNOS status 1A or 1B (the highest categories and most at risk if not urgently transplanted). A recent study found that stable LVAD patients had significantly less 30-day risk of events compared to other status 1A patients concluding that allowance of 30 days of elective status 1A time should not be allocated to stable registrants with implanted LVADs [18]. As VAD technology improves, further revisions to the allocation system will need to be recommended.
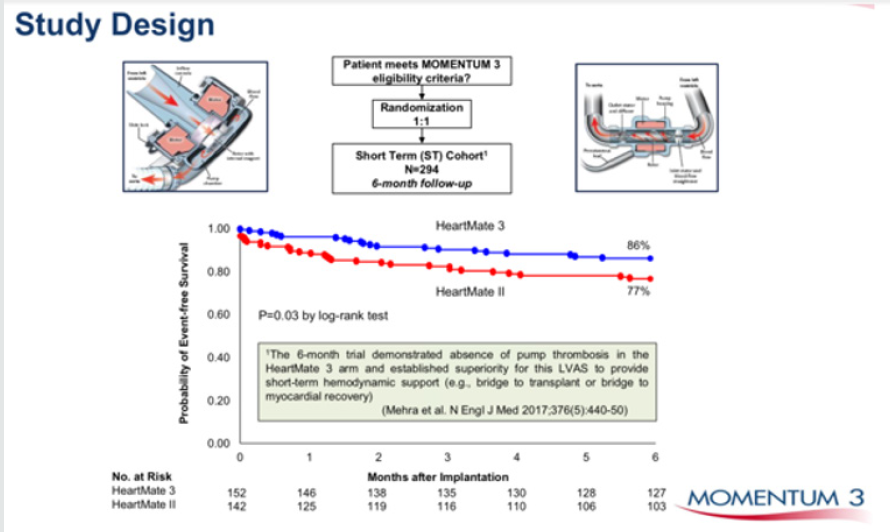
Figure 3: Heartmate 3, the latest centrifugal blood pump in comparison to Heartmate II an axial flow pump. Superior event
free survival is seen with HM 3.
INTERMACS Profile and Description and Timescale to MCS
a) “Crashing and burning”—critical cardiogenic shock. Within hours
b) “Progressive decline”—inotrope dependence with continuing deterioration. Within a few days
c) “Stable but inotrope dependent”—describes clinical stability on mild-to-moderate doses of intravenous inotropes (patients stable on temporary circulatory support without inotropes are within this profile). Within a few weeks
d) “Recurrent advanced heart failure”—“recurrent” rather than “refractory” decompensation. Within weeks to months
e) “Exertion intolerant”—describes patients who are comfortable at rest but are exercise intolerant. Variable
f) “Exertion limited”—describes a patient who is able to do some mild activity but fatigue results within a few minutes of any meaningful physical exertion. Variable
g) “Advanced NYHA III”—describes patients who are clinically stable with a reasonable level of comfortable activity, despite history of previous decompensation that is not recent. Not a candidate for MCS
INTERMACS = Interagency Registry for Mechanically Assisted
Circulatory Support; MCS = mechanical circulatory support;
NYHA = New York Heart Association.
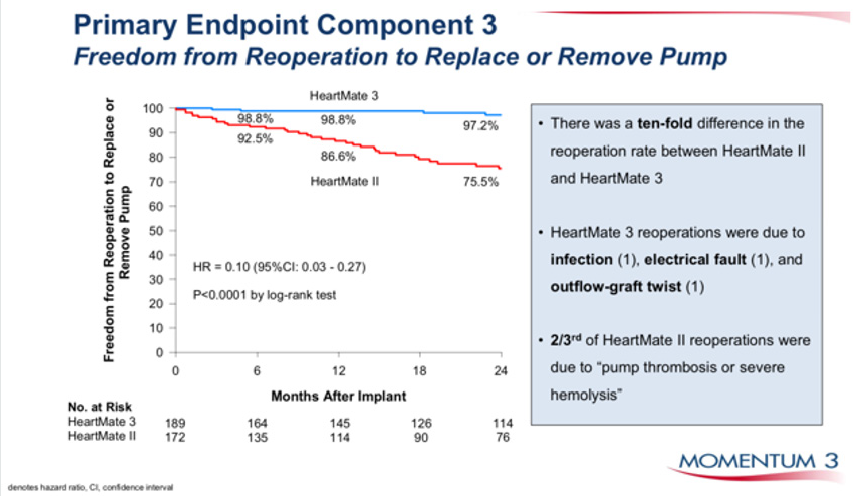
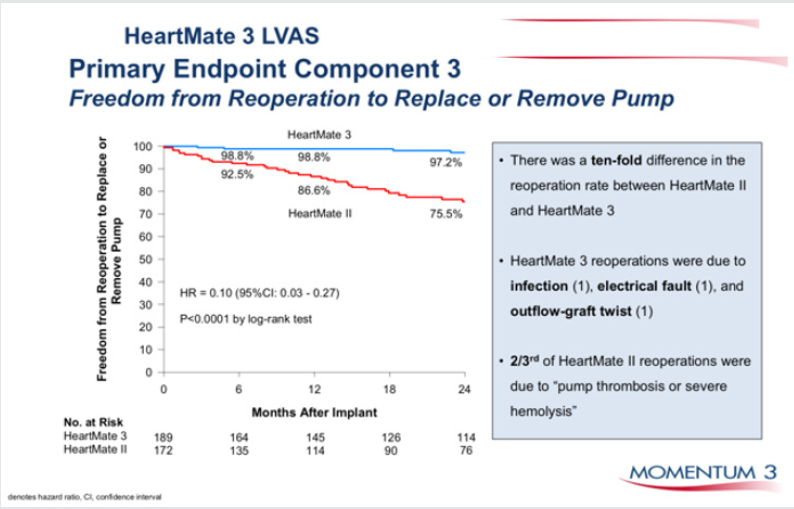
Figure 5: Durability of HM 3 vs HM II, freedom from pump replacement due to pump thrombosis or haemolysis.
Temporary MCS are available that can be implanted quickly and
simply to normalise cardiac output in patients with severe acutely
decompensated heart failure. The CentriMag [19], Tandem Heart
[20], Impella [21] and Circulite [22]. Clinical trials suggest that
treatment of temporary VADs does not necessarily correlate with
better survival, but merely comprise a component of treatment
leading to recovery, upgrade to fully implantable systems as a
bridge to transplant or destination therapy, or transplantation
[23,24]. Device miniaturisation, without externalized drive-lines
connecting the device to a console and longer endurance will be
the future trend of mechanical design for long term support. Blood
pumps with magnetically levitated rotors has shown satisfactory
1-year survival [25]. The smaller size and weight of the continuousflow
devices has allowed an extension of the new VADs into smaller
patients. Fully wireless resonant coupling power sources are
currently undergoing evaluation, which if successful will greatly
reduce the incidence of drive line infections (Figure 5), which is
the weakest point of the technology of current fully implantable
systems. There is some evidence that fully implantable systems will
be available in the near future to greatly improve the quality of life
and to reduce the frequency of severe infections with continuous
flow LVADS.
Many recent studies have focused on the reversed molecular and cellular alterations, such as improved β-adrenergic responses and decreased calcium-regulating gene expression (Figure 6), in patients using LVAD as a bridge to recovery therapy [26]. Functional recovery has been observed in a subset of heart failure patients [26,27]. Recently, a clinical trial using clenbuterol (β-2 agonist and anabolic agent) and LVAD in refractory non-ischemic heart failure patients, reported recovery of heart function in 60% of patients (n=20) with non-ischemic cardiomyopathy that allows the pump to be explanted (Harefield Recovery Protocol Study for Patients with Refractory Chronic Heart Failure, HARPS) [28]. LVAD therapy is associated with decreased collagen turnover and crosslinking and increased tissue angiotensin II. LVAD combined with angiotensinconverting enzyme inhibition results in decreased tissue angiotensin II and collagen cross-linking, normalizes left ventricular end-diastolic pressure volume relationships and is associated with modestly higher rates of bridge to recovery [29]. Other adjunctive treatments including other medications, cell or gene therapy with over expression of SERCA2a might in conjunction with VAD support provide a meaningful alternative therapy in patients with severe heart disease [30].
Conclusion
https://lupinepublishers.com/cardiology-journal/pdf/ACR.MS.ID.000125.pdf
https://lupinepublishers.com/cardiology-journal/abstracts/the-current-status-of-continuous-flow-left-ventricular-assist-devices.ID.000125.php
For
more Lupine Publishers Open
Access Journals Please visit our website
https://www.lupinepublishers.com/
For more Journal of Cardiology & Clinical Research Please Click Here:
For more Journal of Cardiology & Clinical Research Please Click Here:
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online
No comments:
Post a Comment